Modular vs. End-to-End Automation in Cell and Gene Therapy Manufacturing: Finding the Right Fit

The field of cell and gene therapy manufacturing is revolutionising medicine, offering the potential to treat and even cure diseases once considered intractable. As these therapies move from research to commercialisation, the need for robust, scalable, and cost-effective manufacturing processes becomes paramount. Automation plays a critical role in achieving these goals, and two primary approaches have emerged: modular and end-to-end automation.
This blog is the first in a series exploring the nuances of automation in the cell therapy manufacturing process. Here, we compare modular and end-to-end (integrated) systems, examining their respective strengths and weaknesses across key aspects of the manufacturing workflow. This will also illustrate the steps and choices we made in designing Constellation, to ensure a seamless route to industrialised cell and gene therapy manufacturing.
What Do We Mean by Modular Automation and End-to-End Automation?
In the context of cell and gene therapy manufacturing, automation is a commonly used term which can cause some confusion. Within this article, we will refer to the primary forms of automation: modular and end-to-end.
End-to-end systems are typically integrated, box-like, self-contained solutions that aim to encapsulate the entire manufacturing process, within a single closed unit. These systems promise simplicity and sterility through a unified consumable and interface.
In contrast, modular automation integrates individual instruments that each perform distinct unit operations. Note, these instruments themselves may differ in their level of automation and, in some cases, combine multiple steps into one device.
However, a third approach leverages robotics to automate the connections and handling. A modular robotic ecosystem (such as Cellular Origins’ Constellation) brings existing instruments together under a coordinated, automated workflow, automating sterile connections and handling steps between instruments. This enables full-process automation from starting material through to final formulation.
With these fundamental approaches defined, let’s look at the advantages and disadvantages of each.
Connections and Disconnections: Labour and Sterility
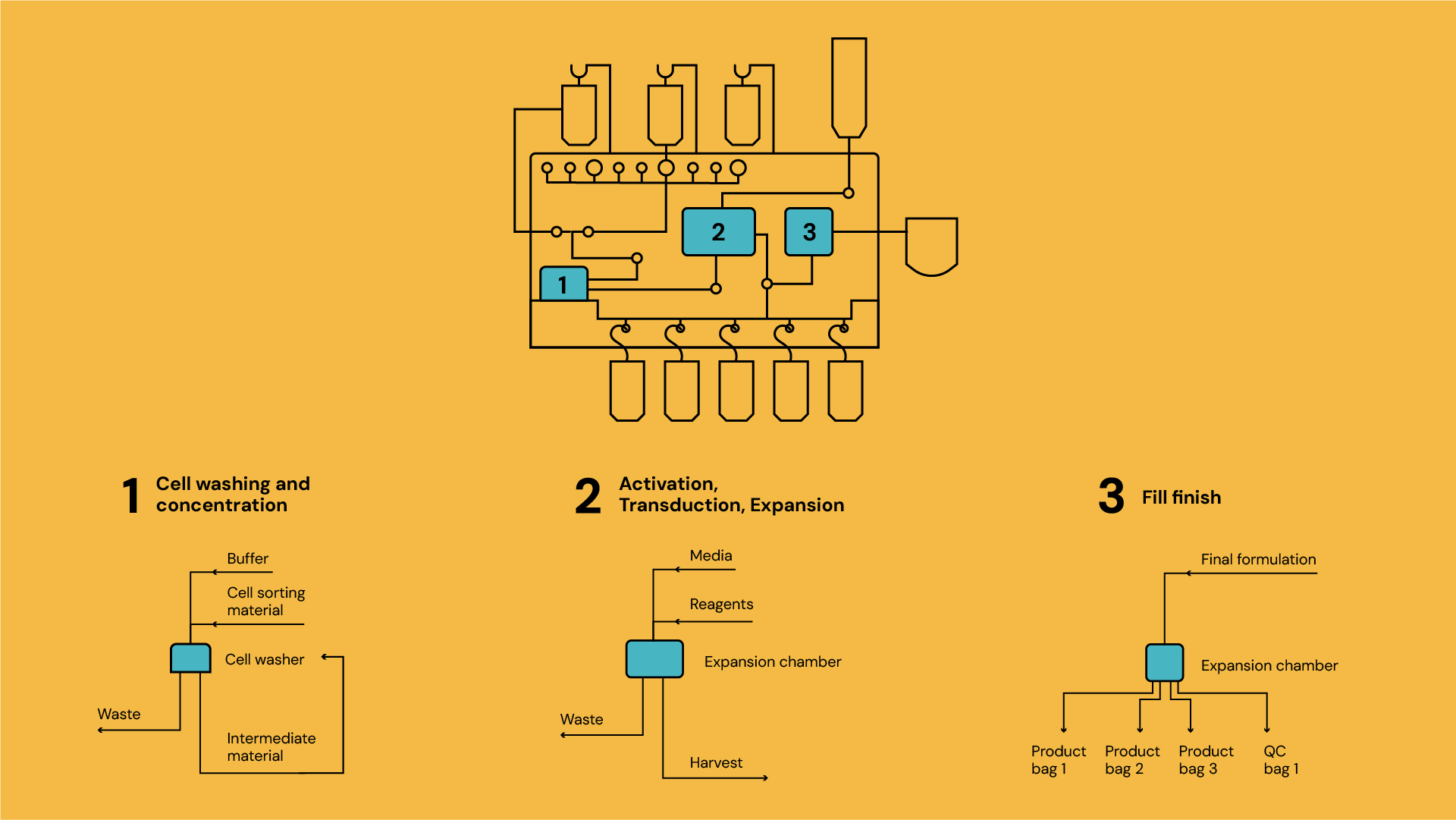
Diagram 1: Cell and gene therapy process steps
One of the most touted benefits of end-to-end automation is its promise of a largely closed system, minimising manual interventions and the associated risks. The concept of a single-use consumable encompassing the entire manufacturing process (from starting material to final product) is certainly appealing. It reduces the number of in-process connections and disconnections, enabling a more “walk-away” approach for manufacturing personnel.
However, even the most integrated end-to-end systems aren’t entirely connection-free. Sampling for quality control and the introduction of or temperature or time-sensitive reagents, like lentivirus, often necessitate operator involvement. While an end-to-end system might boast around 15 connections throughout the process, a modular approach can require 30, as material moves between different unit operations.
This increased number of connections in modular systems raises concerns about sterility. Each connection point represents a potential breach in the closed system and a risk of contamination. Furthermore, the manual execution of these connections adds to the overall labour burden.
Conversely, while end-to-end automation aims to minimize connections, the complexity of the single-use consumable often necessitates sterile connections during its initial setup. The key difference lies in the frequency and timing of these connections throughout the manufacturing process.
Ultimately, while end-to-end systems can reduce the number of in-process connections, this benefit comes with trade-offs in other areas, as we will explore further.
Consumable Complexity: Cost and Reliability
The drive for fewer connections in end-to-end systems often leads to significantly more complex single-use consumables. These aren’t just a sum of individual components but involve intricate networks of tubing, chambers, and integrated sensors. Manufacturing these with high reliability and cost-efficiency presents significant challenges.
Innovations such as manifold blocks offer potential solutions, however many end-to-end systems still rely on complex branching tubing networks. While these may be packaged in user-friendly casings, the complexity is merely transferred from the GMP facility to the consumable supplier. Any reliability issues or difficulties in consumable loading can cause production delays and increased costs.
In contrast, modular automation typically employs simpler, more standardised consumables for each individual unit operation. Although this means a greater number of individual consumables, their simplicity can enhance reliability and reduce costs due to established manufacturing processes.
Load Balancing and Efficiency: Capital and Space
Cell therapy manufacturing processes vary significantly in duration. Some steps, (fluidic transfers, mixing, cell counting), take minutes, while others, e.g. centrifugation, magnetic selection, and filtration, require an hour. Crucially, incubation steps (activation, transduction, expansion) can span hours to days.
Traditional manufacturing lines are designed to balance machine capacity with process bottlenecks. In cell therapy, this would mean ensuring sufficient incubation capacity relative to thawing, for example. End-to-end systems, however, present a challenge: all components—including centrifuges and magnetic separators—remain occupied throughout the entire process, even when idle during long incubation steps. This results in capital inefficiency and larger facility footprints, as each batch requires a dedicated “mini-factory” regardless of the actual utilisation of individual components. E.g. an end-to-end system might occupy 1m³, a bioreactor within a standard incubator requires only 0.01 m² floorspace, despite incubation making up 80% of a 7-day process.
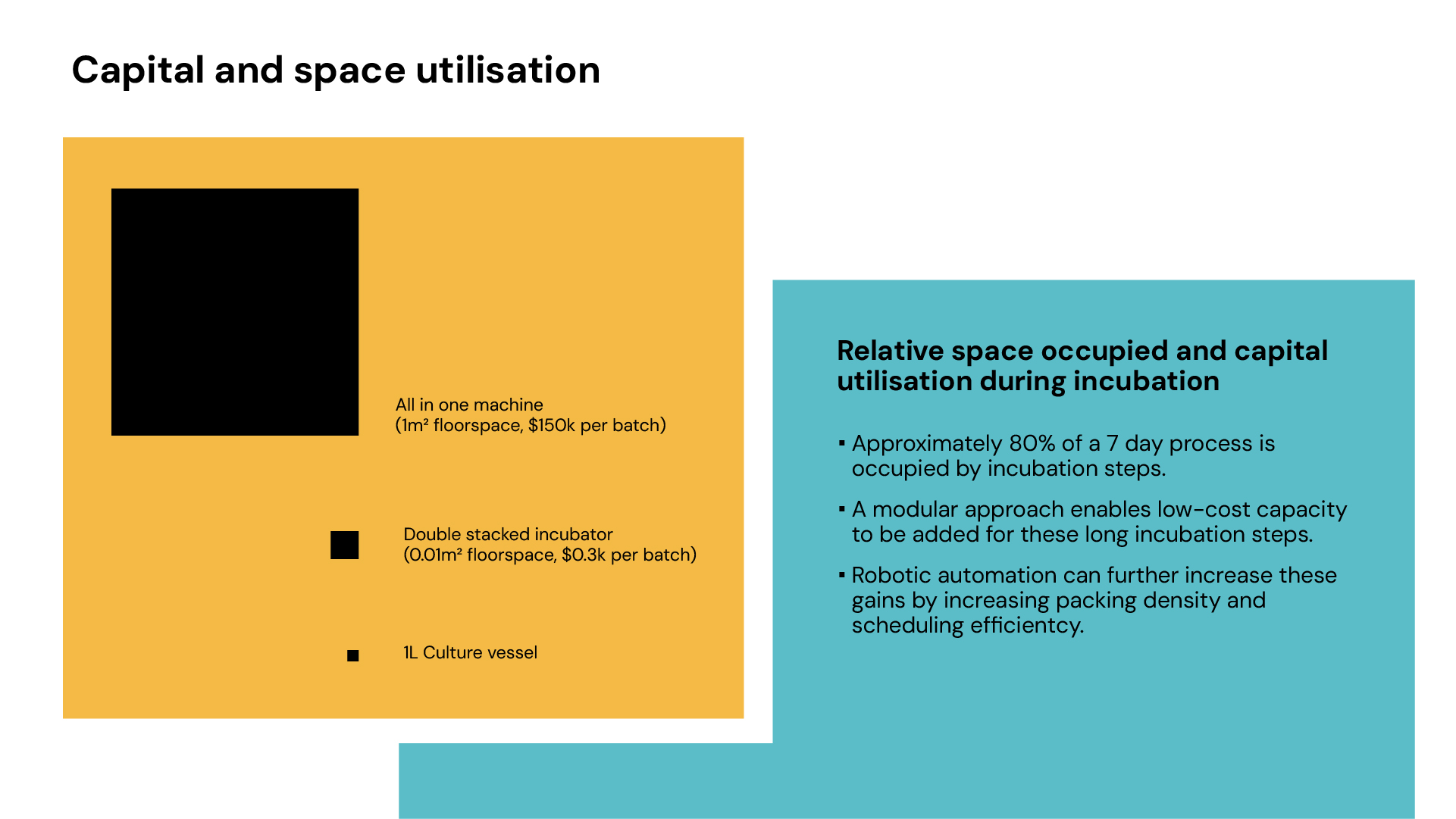
Diagram 2: Comparison of space utilisation
Modular systems enable flexible scaling of unit operations, adding incubators without requiring more centrifuges or cell selection devices, improving capital and space efficiency. They also enhance fault tolerance, allowing replacement or bypass of failed units, unlike end-to-end systems where a single failure can halt the entire batch.
For small-scale production, an end-to-end system may initially appear space-efficient. However, as production volumes increase, the inefficiencies of tying up expensive equipment for extended periods become more apparent. A useful analogy can be found in the diagnostics industry: modular production lines dominate when turnaround time isn’t critical, whereas end-to-end systems are common in point-of-care settings.
Flexibility and Process Optimisation

The inherent integration of end-to-end systems can limit flexibility in process development and optimization. To simplify consumable and capital complexity, manufacturers often standardise core technologies across multiple process steps, even if more optimal tools exist.
Modular automation however empowers developers to choose the most appropriate technology for each step of the cell therapy manufacturing process. This enables greater customisation and optimisation, leading to potential improvements in product quality, yield, and process efficiency. Additionally, modular systems allow for gradual process evolution – swapping/upgrading individual unit operations without requiring a complete platform overhaul.
Choosing the Right Automation Strategy
| Feature | Automated Modular Instruments + Manual Handling | Automated End-to-End Systems | Automated Robotic Ecosystem - Constellation (Automated Modular Instruments + Robotic Handling) |
|---|---|---|---|
| Automates full process | 
| 
| 
|
| Choice of instrument at each process step | 
| 
| 
|
| Reduces manual handling | 
| 
| 
|
| Reduced manual sterile connections | 
| 
| 
|
| Capital and space efficient | 
| 
| 
|
| Flexible architecture | 
| 
| 
|
Both modular and end-to-end automation offer distinct advantages and challenges in cell and gene therapy manufacturing. End-to-end systems aim to streamline workflows and reduce contamination risks by minimising manual connections but comes with increased consumable complexity, reduced capital and space efficiency, and limited flexibility for process optimisation.
Conversely, modular automation (while requiring more in-process connections and labour) provides greater flexibility, cost and space efficiency, and the ability to integrate best-in-class technologies for each process step.
Using a robotic ecosystem, like Constellation which uses robotics to automate the connections and handling between modular instruments, effectively combines the strengths of both methodologies: the flexibility and efficiency of modularity plus the reduced manual handling and process control closer to the ideas of end-to-end systems.
While the hybrid robotic ecosystem offers a compelling path forward, its success hinges on reliably automating the numerous connections inherent in a modular framework. These connection points, traditionally a major source of manual labour and contamination risk in modular setups, must be addressed robustly.
The increased number of manual connections/disconnections in modular systems leads us to our next blog topic: Automated Sterile Connections. We will explore the various methods and technologies available for automating these critical steps, examining their impact on sterility assurance, labour reduction, and overall process efficiency in modular cell therapy manufacturing.
Constellation
Constellation is a fully automated, mobile robotic platform enabling seamless scale-up from clinical to commercial cell therapy manufacturing. It integrates modular, proven third-party instruments and automates all critical GMP steps, with a unified digital layer for real-time monitoring and full traceability. By scaling only when needed, it reduces regulatory risk and labour reliance. We also support facility design and strategy to help developers confidently industrialise manufacturing.

