Flexible cell therapy manufacturing with Constellation
Scale smart with the tools and processes you already trust.
Constellation is a mobile robotic ecosystem for scalable cell therapy manufacturing. Constellation combines advanced mobile robotics, configurable workstations housing 3rd party technologies with full digital integration and sterile transfer technology to enable modular factories.
30X
Space efficient
16X
Reduction in labour
51%
Reduction in cost of goods
Flexible, scalable cell therapy manufacturing built for you
Constellation is a mobile robotic system for scalable CGT production combining modular cell therapy manufacturing with advanced robotics, configurable workstations housing 3rd party technologies with full digital integration and sterile transfer technology to enable modular factories. Constellation’s mobile robots transport consumables and reagents between workstations, and perform kit set up and sterile transfer. Each workstation performs closed process steps, while a unified digital layer connects everything, ensuring full integration, consistency, and control.
Flexible, scalable cell therapy manufacturing built for you
Constellation is a mobile robotic system for scalable CGT production combining modular cell therapy manufacturing with advanced robotics, configurable workstations housing 3rd party technologies with full digital integration and sterile transfer technology to enable modular factories. Constellation’s mobile robots transport consumables and reagents between workstations, and perform kit set up and sterile transfer. Each workstation performs closed process steps, while a unified digital layer connects everything, ensuring full integration, consistency, and control.

Reduce labour. Maximise space
Constellation minimises your manufacturing footprint and maximises labour efficiency, delivering smarter operations with reduced complexity.
24/7 cell therapy manufacturing operation
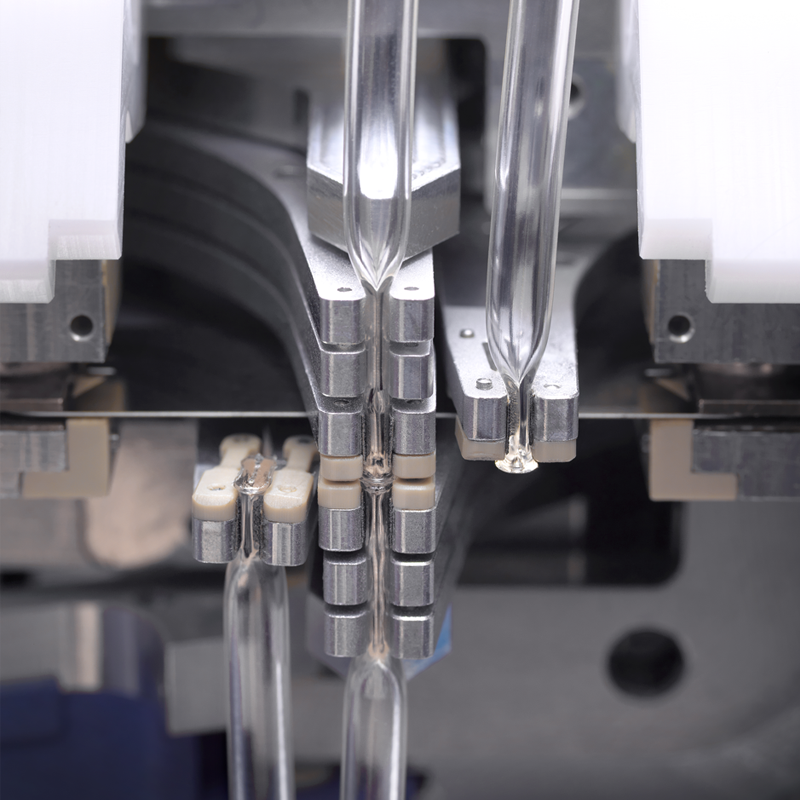
With sterile welding and mobile robots, people can work seamlessly alongside the technology. When maintenance is needed, only the specific process is paused, ensuring the rest of your operations keep running smoothly.

Scale to suit your needs
Constellation’s modular mobile robotic ecosystem lets you expand flexibly—adding hardware only when and where it’s needed. Scale efficiently without being locked into rigid systems.
Adopt at any stage
Whether you’re at clinical trial or commercial scale, Constellation integrates seamlessly into your workflow, offering smooth transition, unparalleled efficiency and ease.


Reduce labour. Maximise space.
Constellation minimises your manufacturing footprint and maximises labour efficiency, delivering smarter operations with reduced complexity.

24/7 cell therapy manufacturing operation
With sterile welding and mobile robots, people can work seamlessly alongside the technology. When maintenance is needed, only the specific process is paused, ensuring the rest of your operations keep running smoothly.

Scale to suit your needs
Constellation’s modular mobile robotic ecosystem lets you expand flexibly—adding hardware only when and where it’s needed. Scale efficiently without being locked into rigid systems.

Adopt at any stage
Whether you’re at clinical trial or commercial scale, Constellation integrates seamlessly into your workflow, offering smooth transition, unparalleled efficiency and ease.
De-risking cell therapy manufacturing
Cell therapy manufacturing is labour-intensive, costly, and prone to errors. Disconnected technologies and manual processes cause variability, limit traceability, and hinder scaling. Furthermore, adding new technologies during commercialisation creates regulatory delays, impacting patient access.
Constellation transforms manufacturing by minimising risks during scale-up. This mobile robotic system for scalable CGT production unites proven technologies, improving efficiency and accelerating therapies to market while maintaining precision and sterility. Advanced robotics and sterile welding automate processes, ensuring compliance and eliminating variability, contamination, and inefficiencies. Its modular design fits into existing facilities without costly revalidation, reducing labour and operational costs. A unified software layer ensures control, traceability, and consistent outcomes. Mobile robots enable flexible, 24/7 operation in Grade C/D spaces, with built-in redundancy ensuring smooth, reliable operations even during maintenance or equipment replacement.
With Cellular Origins, scaling is no longer a challenge—it’s an opportunity to achieve smarter, safer, and faster manufacturing.
De-risking cell therapy manufacturing
Cell therapy manufacturing is labour-intensive, costly, and prone to errors. Disconnected technologies and manual processes cause variability, limit traceability, and hinder scaling. Furthermore, adding new technologies during commercialisation creates regulatory delays, impacting patient access.
Constellation transforms manufacturing by minimising risks during scale-up. This mobile robotic system for scalable CGT production unites proven technologies, improving efficiency and accelerating therapies to market while maintaining precision and sterility. Advanced robotics and sterile welding automate processes, ensuring compliance and eliminating variability, contamination, and inefficiencies. Its modular design fits into existing facilities without costly revalidation, reducing labour and operational costs. A unified software layer ensures control, traceability, and consistent outcomes. Mobile robots enable flexible, 24/7 operation in Grade C/D spaces, with built-in redundancy ensuring smooth, reliable operations even during maintenance or equipment replacement.
With Cellular Origins, scaling is no longer a challenge—it’s an opportunity to achieve smarter, safer, and faster manufacturing.
Clean room render based on Cell and Gene Therapy Catapult Stevenage Manufacturing Innovation Centre design.
Industrialising cell therapy manufacture today and beyond
Constellation goes beyond scaling – it shapes the future. Constellation eliminates the need for extensive infrastructure changes, empowering developers to scale flexibly and cost-effectively.
Our adaptable ecosystem, integrating mobile robotics with proven technologies ensures consistent quality and compliance across locations while reducing costs and accelerating time to market. By enabling modular cell therapy manufacturing with advanced robotics, Constellation de-risks scale-up and simplifies operations, allowing developers to focus on what matters most: innovation and meeting the growing demand for life-saving therapies.
Constellation is more than a solution- it’s a partnership for progress.
Industrialising cell therapy manufacture today and beyond
Constellation goes beyond scaling – it shapes the future. Constellation eliminates the need for extensive infrastructure changes, empowering developers to scale flexibly and cost-effectively.
Our adaptable ecosystem, integrating mobile robotics with proven technologies ensures consistent quality and compliance across locations while reducing costs and accelerating time to market. By enabling modular cell therapy manufacturing with advanced robotics, Constellation de-risks scale-up and simplifies operations, allowing developers to focus on what matters most: innovation and meeting the growing demand for life-saving therapies.
Constellation is more than a solution – it’s a partnership for progress.
Want to find out more?
eBook | Industrialising cell and gene therapy manufacturing
With contributions from Cytiva, Fresenius Kabi, Cell and Gene Therapy Catapult, Thermo Fisher and ScaleReady, this guide uncovers the challenges in scaling CGT manufacturing and examines the role of automation and robotics to reduce cost of goods, and meet regulatory and quality standards. Download the eBook…
Video | Advanced Therapies Weeks 2025 stand presentations
Watch our stand presentations from Advanced Therapies Week 2025 where Cellular Origins and our leading technology partners join together to discuss why collaboration is so important to industrialise cell therapy manufacturing. Watch the videos…
Blog | First customer installation of Constellation at Cell and Gene Therapy Catapult
The testbeds, located at Cell and Gene Therapy Catapult’s Stevenage Manufacturing Innovation Centre, are among the first sandbox environments in the world. Read more…
Whitepaper | How to de-risk cell and gene therapy manufacturing
Uncover ways to de-risk scale up whilst boosting space efficiency by 30%, cutting labour costs 16x, and lowering production costs by 51%. Download the whitepaper…
Ready to transform your cell therapy manufacturing?
Take the first step to scalable, efficient, and future-ready cell therapy manufacturing. Complete the form below and one of our experts will be in touch.
Let’s shape the future together.
Ready to transform your cell therapy manufacturing?
Take the first step to scalable, efficient, and future-ready cell therapy manufacturing. Complete the form below and one of our experts will be in touch.
Let’s shape the future together.
Your questions answered
How does Constellation guarantee precise control, consistency and quality when manufacturing at scale?
Constellation delivers unparalleled process consistency and product quality by combining precision robotics, sterile welding, and closed-system automation. Unlike traditional manual processing, which is inherently variable, Constellation ensures highly repeatable, controlled operations across every batch.
A key differentiator is our sterile welding capability, which enables secure, aseptic fluid transfers within a fully closed system. This eliminates reliance on open processing, meaning Constellation can operate reliably in lower-classified C/D cleanroom environments while maintaining a Grade A sterile environment at the point of processing.
By removing human variability and automating critical process steps such as media exchanges, cell culture handling, and fluid transfers, Constellation significantly reduces contamination risks and batch failures, ensuring high cell viability, sterility, and product consistency at scale. The system also provides real-time process monitoring and automated adjustments, further safeguarding quality and ensuring reproducible outcomes.
When should we begin to think about Automation? When is the right time to implement it?
Cell therapy developers should start thinking about automation early – future-proofing manufacturing strategies, reducing long-term regulatory hurdles, and de-risking scale-up. However, you shouldn’t feel under any pressure to implement early. Do so when the timing is right for you.
Constellation provides complete flexibility – you can adopt automation at any time and at the pace that suits your development timeline. Unlike fixed, inflexible systems that lock you into a specific process, Constellation allows you to scale seamlessly from clinical to commercial manufacturing without process changes. This means you can continue refining your process manually while keeping automation in mind, knowing that Constellation can integrate when the time is right.
With Constellation, you retain full control over when and how you automate, ensuring your technology and processes evolve on your terms.
Will we need to revalidate our processes if we integrate Constellation?
Constellation is designed for regulatory compliance from the ground up. It maintains GMP-aligned processing and provides comprehensive electronic batch records (EBR) for full traceability. By mirroring manual operations within a controlled, automated system, Constellation standardises processes, minimising deviations and risk of errors, and can be introduced without requiring significant process revalidation—accelerating the path to commercialisation without regulatory roadblocks.
Can Constellation adapt to our current processes or do we have to change them around Constellation?
Constellation is not a fixed, rigid system. It is a flexible automation platform which combines your existing, proven technologies and processes and automates them within one unified hardware and digital layer.
Our mobile robots transport product, consumables and reagents between workstations housing proven third-party technologies, operating those unit operations and automating all critical GMP manufacturing steps. A unified digital layer ensures seamless integration, real-time monitoring, and full traceability.
By using existing, regulatory-approved technologies, Constellation enables rapid scale-up only when needed – lowering regulatory risk, reducing labour, maximising facility efficiency, and de-risking the journey to commercial-scale manufacturing. It is a truly flexible and adaptable option to scale to suit you and your requirements.
Will I have to build a new facility to scale up commercial manufacturing?
Not necessarily. You can deploy our solution within your existing facility or opt to build a new one. It entirely depends on your objectives, stage of development, and commercial ambitions.
Constellation is designed to provide flexibility. It can integrate into established environments or form the foundation of a purpose-built facility optimised for scale. Many organisations choose to begin within their current infrastructure to develop and refine their processes without overcommitting capital too early. This allows teams to focus on achieving process robustness and clinical success before scaling into a larger, more permanent manufacturing setup.
When you’re ready, Constellation enables a seamless transition into a full-scale, modular factory model, without needing to redesign your process. This approach minimises risk, maximises efficiency, and ensures you only invest in new infrastructure when you’re confident in your manufacturing process and market trajectory. In short, it’s about what’s right for your journey: start where you are, scale when you’re ready.
We have a highly skilled team of operators and scientists who are experts in our process. If we introduce automation, how can we ensure that it enhances their capabilities rather than replacing their critical decision-making?
Constellation is designed to enhance, not replace, operator expertise. While manual processes introduce variability and reliance on skilled technicians, Constellation standardises key process steps while allowing operators to focus on process oversight, decision-making, and innovation. Real-time monitoring and intuitive operator interfaces ensure that teams maintain full visibility and control over the manufacturing process, allowing manual intervention alongside automation if necessary, without interrupting the workflow.
How does Constellation de-risk our manufacturing process, ensuring reliable and scalable production without unexpected failures?
Constellation is specifically designed to de-risk cell therapy manufacturing by eliminating the most common failure points associated with manual processing. By integrating precision robotics, closed-system automation, and sterile welding, Constellation reduces contamination risk, process variability, and reliance on skilled labour – three of the biggest challenges in cell therapy production.
Unlike traditional manual workflows, where human intervention increases the likelihood of errors and inconsistencies, Constellation provides standardised, repeatable execution at every stage. Furthermore, real-time monitoring and digital batch recording within one unified digital layer provides complete traceability, allowing for proactive issue detection and seamless regulatory compliance.
Crucially, Constellation has been engineered with practical flexibility in mind. If a unit requires maintenance or unexpected attention, human operators can safely intervene in that specific area without needing to shut down the entire process. This modular design not only ensures operational continuity, but also adds a vital layer of resilience and confidence in manufacturing uptime.
What’s the real cost of implementation? How does this compare to traditional manual processes over time, factoring in maintenance, training, and operational efficiencies?
Constellation delivers a measurable return on investment, often from the earliest stages of deployment, by reducing reliance on highly skilled labour, increasing production throughput, and minimising batch variability. It’s a capital-efficient solution that aligns with both early-stage clinical needs and commercial scalability.
Unlike traditional manufacturing setups that require large manual teams, extensive training programmes, and expansive cleanroom footprints, Constellation enables a leaner, more efficient, and space-optimised operational model. Over time, automation drives down cost per dose, accelerates time to market, and improves facility utilisation, ensuring long-term cost-effectiveness without compromising process control or compliance.