Why a cell therapy factory isn't a car plant

Moving beyond traditional automation: How mobile robots provide the flexibility cell therapy demands
For cell therapy to deliver on its promise, scaling from small, manual laboratory environments to large-scale manufacturing is critical. Yet industrialising these highly personalised, biologically complex therapies presents unique challenges, unlike those faced by traditional mass-manufactured products. Any successful approach must directly address the inherent variability, stringent regulatory requirements, and critical reliability demands that define this emerging field.
In this blog, we explore how advanced manufacturing principles – (such as flexibility, adaptability, and modularity), that are being rapidly adopted in other industries ranging from silicon fab, to lab automation, and yes modern car automation – can help overcome these distinct challenges. Success at scale depends on selecting the right automation approach. We’ll examine the critical factors that make automation effective and outline the capabilities cell therapy facilities need to meet both today’s demands and tomorrow’s expectations.
Why traditional automation falls short in cell therapy manufacturing
The challenge of rigid scheduling
Traditionally, automation is designed around predictable, time-bound operations performed in sequence. The traditional automotive production line, as exemplified by Henry Ford and the Model T, revolutionised car manufacturing and is a masterpiece of predictable, linear flow. Parts move between stations on fixed conveyors, static robots work on specialised workcells to carry out operations. The advantages are clear: well-understood technology, straightforward validation, and efficiency for scaled, repetitive operations.

Illustration of traditional linear manufacturing approach in the automotive industry
While some raw materials can be cryopreserved and scheduled in advance, fresh patient-derived cells often arrive at unpredictable times due to clinical constraints. The duration of steps such as cell expansion or in process QC can fluctuate from one patient batch to the next. Many cell therapy workflows involve repeated steps such as incubation or sampling steps, requiring products to loop back through the process, and some of these steps may be dependent on previous steps such as cell counts. Even with all of these variations, critical process timings must be maintained to ensure cell quality and ultimately therapeutic efficacy.
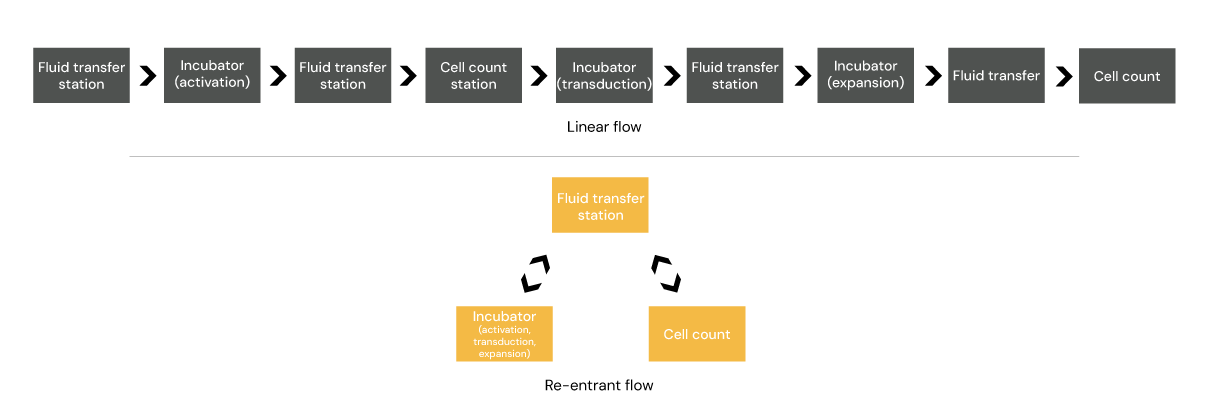
Comparison of part of the cell therapy manufacturing process (activitation to expansion) – traditional linear manufacturing approach v mobile robotics
This inherent variability calls for a shift away from rigid, linear systems, like the Ford approach, where fixed conveyors, individual stations or, even rail-bound robots can quickly become bottlenecks – towards a more dynamic, modular approach designed to adapt in real time.
The most effective automation must be able to handle a non-linear workflow, intelligently routing products to the next available station. One proven solution is the use of Autonomous Mobile Robots (AMRs), which are already staples in lab automation, silicon fabrication and logistics due to their ability to create adaptable production lines.
Another approach, typically for smaller payloads, involves Planar Motor Systems, which create ‘super highways’ of flexible, individually controlled magnetic carriers. Both technologies enable the creation of truly dynamic and adaptive factories, where workflows can be adjusted in real-time to match the unpredictable nature of the science and ensure that an individual batch arrives on time.

Cellular Origins’ Constellation platform using dynamic mobile robotics
The critical need for reliability
In typical manufacturing, downtime is an issue of cost and efficiency. In autologous cell therapy downtime isn’t just an operational setback, it represents the risk of losing irreplaceable patient-specific batches, directly impacting patient outcomes. Therefore, ensuring maximum reliability and uptime is paramount.
Reliability is a critical factor when evaluating any automation architecture. Systems that depend on a single robotic arm or centralised component to manage all tasks introduce a major single point of failure. One fault can compromise the entire process.
For context, a typical industrial robot has a Mean Time Between Failures (MTBF) of 4.5 years. While that may seem high, in a facility operating 10 robots, it equates to roughly two failures per year. Even with the most robust robotics employed, there will always be a single point of failure, and in more complex systems with multiple actuators and controllers, the risk of disruption increases even further.
A more resilient approach is a distributed, fault-tolerant system, a principle mastered in semiconductor manufacturing. In a silicon fab, if an automated transport vehicle fails, the central control system instantly reroutes others to bypass the issue, ensuring multi-million dollar tools never sit idle. Similarly, in a cell therapy layout where multiple, independent robots service a series of modular stations, the failure of a single robot or module does not stop production. The system can simply re-route tasks to the other operational units, ensuring the manufacturing process continues. This significantly de-risks the operation and provides the robust reliability that patient safety demands.
Example of dynamic mobile robots used in silicon fab manufacturing
Navigating cleanroom constraints
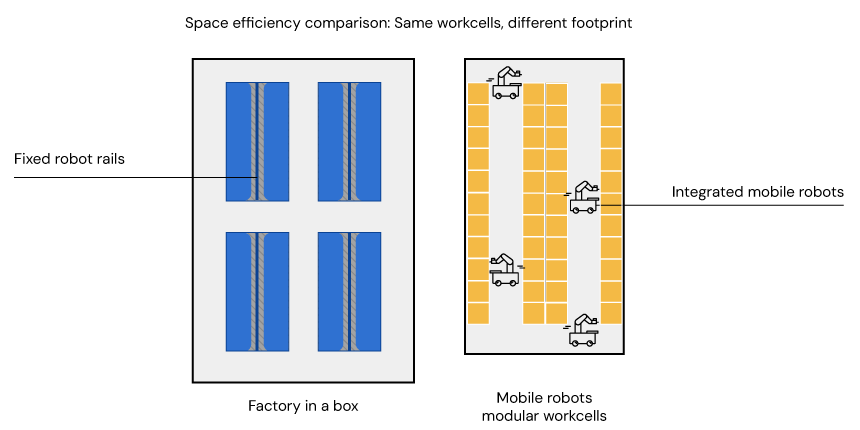
While a factory-in-a-box may appear space-efficient, additional clearance is still required for maintenance and operator access. In contrast, modular work cells served by mobile robots optimise space utilization, as robots can easily move aside to allow access when needed and units can be placed continuously along walls
Manufacturing cell therapies requires the use of GMP-compliant cleanrooms, which are among the most expensive and highly regulated production environments in the world. The cost of building and operating these facilities (ranging from $100 to over $1,500 per square foot) means that every square inch must be used as efficiently as possible.
This presents a challenge for certain automation designs. Large, dense layouts with fixed equipment and complex conveyor mechanisms not only consume valuable floor space but are also difficult to access for the rigorous cleaning and maintenance protocols required by GMP standards.
One option is to pursue a robotic isolator, or a factory-in-a-box , which aims to contain the entire sterile process within an enclosed pod, potentially reducing the need for a large, classified cleanroom. However, many critical ancillary steps, such as material preparation, in-process analytics, or final formulation, often fall outside the pod’s capabilities, and may still require a separate GMP-grade cleanroom environment. Integration with larger or specialised instruments for example cryofreezers may be difficult or impossible. Furthermore, while these enclosed systems appear compact, they often demand significant external clearance for servicing and material loading, which can ultimately lead to less efficient facility layouts than initially anticipated.
An ideal automation layout should be compact, promote efficient movement, and allow for easy access. Modular layouts served by mobile robotics inherently simplify GMP compliance by providing straightforward human access for cleaning, maintenance, and process intervention – critical factors in maintaining high regulatory standards. Systems that allow humans and robots to share the same circulation space, for instance, can dramatically improve the overall efficiency and utility of the cleanroom footprint.
Designing for flexibility and adaptability
Cell therapy is one of the fastest-advancing fields in medicine. The manufacturing processes of today will almost certainly be different from the processes of tomorrow. New tools will be developed, cell engineering techniques will be refined, and new therapeutic modalities will emerge.
This reality makes long-term flexibility and adaptability a critical strategic consideration. Choosing a modular, flexible design not only enables rapid adoption of future innovations but significantly reduces long-term capital expenditure by avoiding costly reconfigurations or obsolescence. Committing to a rigid, enclosed, or “locked-in” automation architecture is a significant strategic risk – making it difficult or even impossible to integrate new technologies, evolve workflows, or scale production without a complete and costly overhaul.

The most strategic approach is to adopt a modular, “Lego®-like” framework. In fact, state-of-the-art automotive plants have evolved far beyond the traditional production line of Henry Ford. Major manufacturers like Audi and Hyundai are now embracing this modular principle by using an ‘unboxed’ approach – flexible assembly islands, linked together by mobile robots, instead of a single, rigid line. This allows them to build multiple car models simultaneously and rapidly reconfigure for future products.


Example of mobile robotics at a Hyundai automotive plant – a mobile robot moves the car chassis toward a Spot inspection robot.
Similarly, a modular cell therapy facility allows for evolution over time. New modules with new technologies can be added, the layout can be reconfigured to optimise for a new process, and production can be scaled by simply adding more modular units. This ensures that the manufacturing facility is not a static monument to a single process, but a dynamic and future-proof asset that can grow and adapt with the science.
Comparing approaches: mobile robots vs traditional fixed systems
| Key Criteria | Mobile Modular Robots | Traditional Linear Automated Solutions |
|---|---|---|
| Process Flexibility | ✅ Seamlessly handles asynchronous workflows, staggered step durations, and variable input timings | ❌ Often requires rigid scheduling, making it difficult to accommodate clinical variability |
| Scalability and Reconfiguration | ✅ Modular design enables rapid scale-up, adaptation, and reorganisation as demand or processes evolve | ⚠️ Expansion may require major redesign or duplication of entire systems |
| Fault Tolerance and Uptime | ✅ Built-in redundancy allows rerouting around failed components; single points of failure don’t halt production | ❌ A failure in a key component can stop the entire process, risking loss of patient batches |
| Cleanroom Compatibility | ✅ Designed for shared human–robot workspaces; easy to access, clean, and maintain | ❌ Dense or enclosed layouts can limit access for cleaning and troubleshooting |
| Integration with Specialist Equipment | ✅ Easily incorporates cryo-storage, analytical systems, and custom instrumentation. Can automate from door to door. | ❌ Integration is often limited by physical constraints or vendor lock-in |
| Complexity of implementation | ⚠️ Requires orchestration via advanced software platforms and more complex validation of dynamic paths and interactions—though these are increasingly standardized in other industries | ✅ Generally simpler initial setup for dedicated, fixed tasks with fewer system interactions, often with pre-configured workflows. |
| Facility Space Efficiency | ✅ Optimises both operational footprint and service access by decoupling movement from fixed infrastructure | ❌ Requires additional clearance or duplicated equipment, reducing spatial efficiency |
| Adaptability to Innovation | ✅ Supports continuous evolution—new modules or technologies can be introduced without disrupting the system | ❌ Less adaptable to new processes; upgrades may be complex and resource-intensive |
Conclusion: Building a resilient factory for cell therapy manufacturing
Ultimately, the goal of industrialising cell therapy is to deliver these life-saving treatments to more patients – safely, reliably, and at scale. Any strategy that can’t evolve with scientific progress, or recover quickly from a component failure, puts both patient safety and commercial viability at risk. The choice of automation architecture is central to that mission.
That’s why a factory-first approach to cell therapy industrialisation is so essential. A modular facility, powered by a fleet of mobile robots, provides the dynamic logistical backbone needed to manage complexity and scale. These robots transport closed, single-use consumables between specialised workcells for cell selection, expansion, fill-finish, and potentially dedicated stations for QC, analytics, or enclosed isolators for open handling steps like vial filling. Critically, these mobile systems shouldn’t stop at the cleanroom. Extending mobile robotics across the broader facility – connecting cleanroom operations with warehousing, waste disposal, and material handling – will become increasingly vital as dose volumes grow.
This isn’t a speculative vision. Amazon, one of the pioneers of mobile robotics, recently deployed its one millionth robot. Billions are being invested globally to make these systems smarter, more robust, and increasingly integrated with AI-driven orchestration.
At Cellular Origins, we’re harnessing that momentum, applying the same proven principles of modularity and dynamic transport that have already reshaped industries like semiconductor manufacturing and advanced lab automation, and yes, even modern car manufacturing which no longer resembles the rigid car plants of Henry Ford. This integrated approach ensures process integrity and reliability for today’s therapies, while creating a future-ready manufacturing platform – engineered to scale, built to adapt, and designed to safeguard each patient’s treatment.
We’re not building a factory for yesterday’s process. We’re building one for today’s therapies at tomorrow’s scale. Flexible. Reliable. Scalable by design. Ready to serve the next hundred thousand patients, and the hundred thousand after that.
Video of Cellular Origins’ Constellation platform using dynamic mobile robotics
Constellation
Constellation is a fully automated, mobile robotic platform enabling seamless scale-up from clinical to commercial cell therapy manufacturing. It integrates modular, proven third-party instruments and automates all critical GMP steps, with a unified digital layer for real-time monitoring and full traceability. By scaling only when needed, it reduces regulatory risk and labour reliance. We also support facility design and strategy to help developers confidently industrialise manufacturing.

